How Many Unpaired Electrons in Selenium
Selenium contains four unpaired electrons in its outermost orbital. How many unpaired electrons does selenium have.
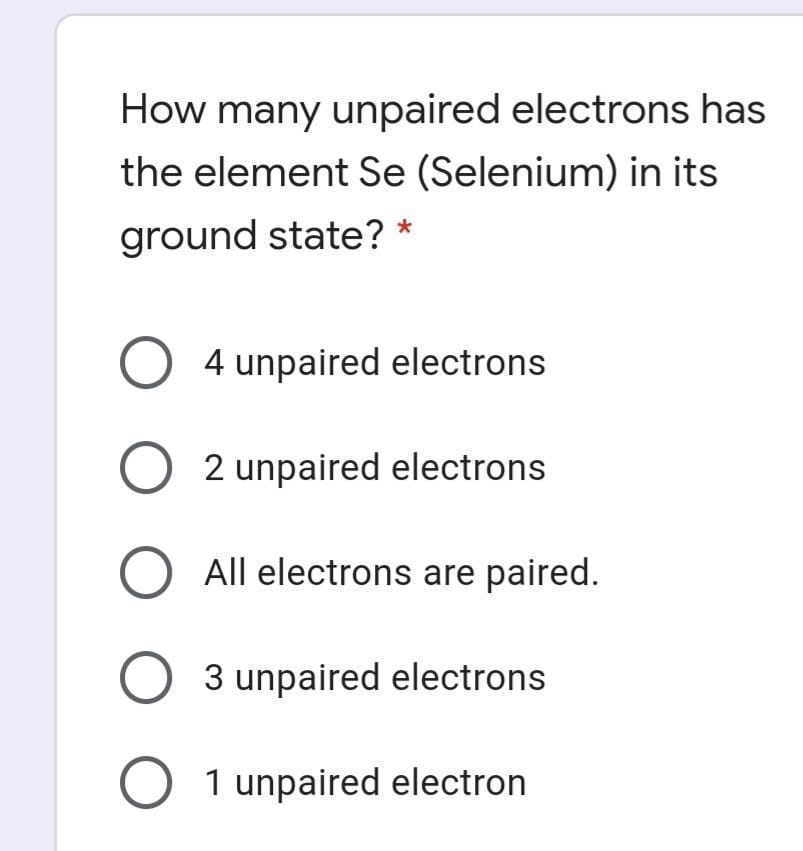
Answered How Many Unpaired Electrons Has The Bartleby
Selenium is a member of the sulfur family with elements including tellurium and polonium.
. Heres why Selenium actually has 2 unpaired electrons in the 4p-orbital which makes it paramagnetic. Selenium contains four unpaired electrons in its outermost orbital. It is located in group 16 below oxygen and sulfur.
Electron configuration of Selenium ends with 4s2 4p4. So there are 3 unpaired electrons. So there are 4 unpaired electrons.
On the periodic table selenium is element number 34. Then you can place the last electron in the first box. These electrons can form bonds with other elements and are called valence electrons.
How many valence shell electrons does boron have. In the ground state Se has 2 unpaired electrons. In this case selenium has six unpaired electrons.
Cl- group 17 ns2np5- 1 unpaired electron. How many unpaired electrons has the element Selenium Z34 in its ground state. This family has six electrons in the outermost shell.
2 unpaired electrons O 1 unpaired electron close Start your trial now. Here selenium has four unpaired electrons. The first is to use the Periodic Table to figure out how many electrons Selenium.
How many unpaired electrons are found in the ground state electron configuration of selenium Se. How many unpaired electrons has the element Se Selenium in its ground state. In your mind picture three boxes and each box holds two electrons But since you have certain rules.
Therefore the valency of selenium is 2. How many electrons does selenium Z 34 have in its valence shell. The six electrons in the outermost shell allow selenium to have a variety of valence numbers.
So you know that P can hold up to 6 electrons. Its electron configuration looks like this SeAr4s23d104p4 Now youd be tempted to say that selenium has no unpaired electrons either but youd be wrong. On the periodic table selenium is element number 34.
Gd3 in accordance with Hunds rule maximum multiplicity contains seven unpaired electrons and is thus strongly paramagnetic 4. As we can see in the d orbital all the electrons are paired hence it has zero unpaired electrons. Not at all Slightly Kinda Very much Completely Still have questions.
Therefore selenium has two unpaired electrons. An atom of which of the following elements is not diamagnetic in the ground state. By looking at the electron configuration of selenium it is possible to determine how many electrons are in each sub-shell.
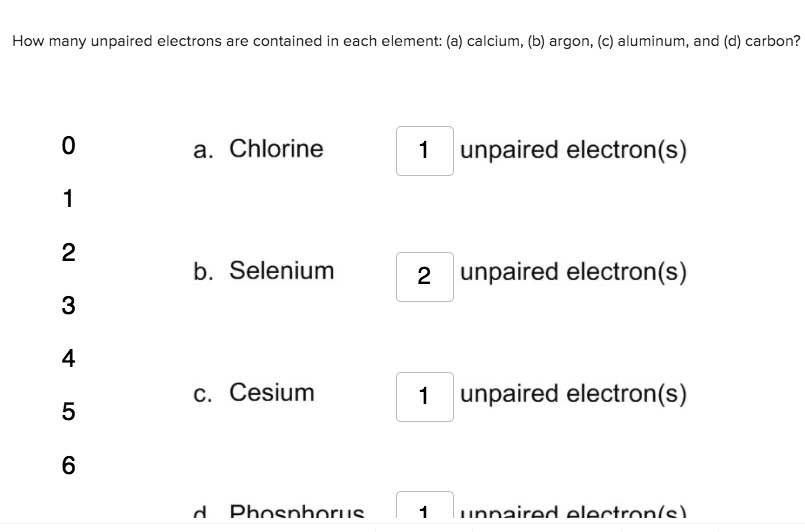
Cesium 1 unpaired electrons 5 6 d Phosphorus 1 unnaired electronic This problem has been solved. There are two ways to find the number of valence electrons in Selenium Se. The six electrons in the outermost shell allow selenium to have a variety of valence numbers.
It is located in group 16 below oxygen and sulfur. 4p can be filled with 6 electrons. I has the largest number of electrons 54 and has the largest inter-electron repulsion making it the largest.
This brings us to selenium Se. See the answer See the answer See the answer done loading. Valency and valence electrons of selenium Se Again the electron configuration of selenium excited state is Se 34 1s 2 2s 2 2p 6 3s 2 3p 6 4s 1 3d 10 4p x1 4p y1 4p z1 4f xy1 4f yz1.
2 unpaired electrons 3 4 c. How many unpaired electrons has the element Selenium Z34 in its ground state. You need to place 1 electron in each of them first.
Cs-group 1 ns1- one unpaired electron. Solution for Consider an orbital diagram of the element selenium Ar 4s2 3d10 4p4. Now imagine 3 boxes for electrons for 4p shell.
Hence out of the given options F e 2 has the most number of unpaired electrons ie. This group of elements is sometimes called the chalcogens. Chemistry questions and answers.
Which of the following has the maximum number of unpaired electrons. By signing up youll get thousands. Find more answers Ask your question.
Se-2 Cl-1 Cs-1 P-1. For finding the number of unpaired electrons then first we have to find the atomic number of the element then write the configuration in the ground state then according to the oxidation state subtract the number of electrons from the outer shell. The correct answer was given.
Ok if you look at the Periodic Table Se is in 3P4. Let us consider the groups the elements belong to. Atomic mass of Selenium is 79.
These boxes should have 2 electrons in each of them. Se- group 16 ns2np4- two unpaired valence electrons. So the valency of selenium is 4.
Selenium atomic 34 34 electrons. The number of unpaired electrons in a gaseous selenium atom is a 2 b 3 c 4 d 5 3 from CHEMISTRY 200 at University of California San Diego So there are 4 unpaired electrons. 4 unpaired electrons 3 unpaired electrons O All electrons are paired.
Se- group 16 ns2np4- two unpaired valence electrons Cl- group 17 ns2np5- 1 unpaired electron Cs-group 1 ns1- one unpaired electron P-group 15- ns2np3- three unpaired valence electrons Survey Did this page answer your question. Selenium specifically has an electron configuration of 2-8-18-6. How many unpaired electrons are found in the ground-state electron configuration of selenium Se.
These electrons can form bonds with other elements and are called valence electrons. Therefore the valence electrons of selenium are six. Atomic Orbital Diagram for Selenium Se Selenium ion Se 2- electron configuration The electron configuration of selenium shows that the last shell of selenium Se has six electrons.
Here selenium Se has two unpaired electrons. Fe has __ that isare unpaired in its d orbitals.

Solved How Many Unpaired Electrons Are Contained In Each Chegg Com

Clicker 1 How Many Unpaired Electrons And Valence Electrons Does Se Have A 0 14 B 2 6 C 2 14 D 0 6 E 2 Ppt Download
Comments
Post a Comment